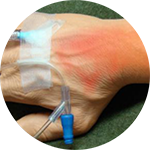
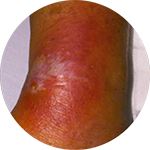
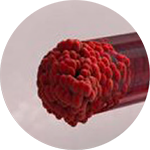
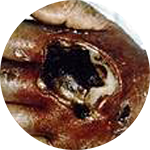
*Compared to an open system
**Compared to a non-blood control catheter
1. Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure. Infus Nurs Society. 2015;38(3):189-203.
2. European Biosafety Network. Prevention of Sharps Injuries in the Hospital and Healthcare Sector. European Biosafety Network Implementation Guidance Toolkit for EU Council Directive 2010/32/EU. January 2013 Available at: www.europeansafetynetwork.eu (accessed May 2017).
3. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs. 2021;44(suppl 1):S1-S224.
4. Canadian Vascular Access Association (2019). Canadian Vascular Access and Infusion Therapy Guidelines. Pembroke, ON: Pappin Communication
5. Haeseler G, Hildebrand M, Fritscher J. Efficacy and ease of use of an intravenous catheter designed to prevent blood leakage: A prospective observational study. J Vasc Access. 2015;16(3):233-236.
6. Onia R, Eshun-Wilson I, Arce C, Ellis C, Parvu V, Hassman D, Kassler-Taub K. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. Current medical research and opinion. 2011 Jul 1;27(7):1339-46.
7. Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheterstabilization systems. J Infus Nurs. 2010;33(6):371-384.
8. González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.