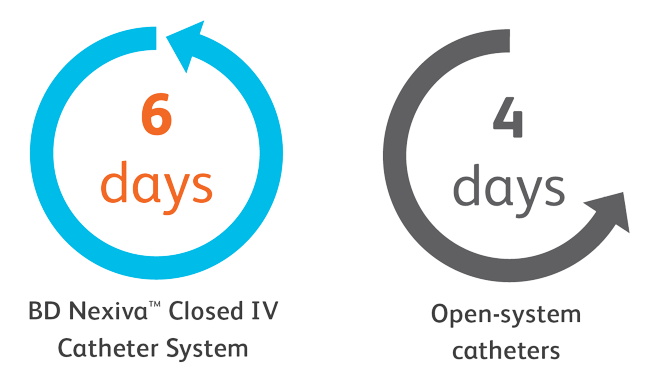
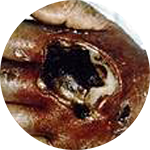
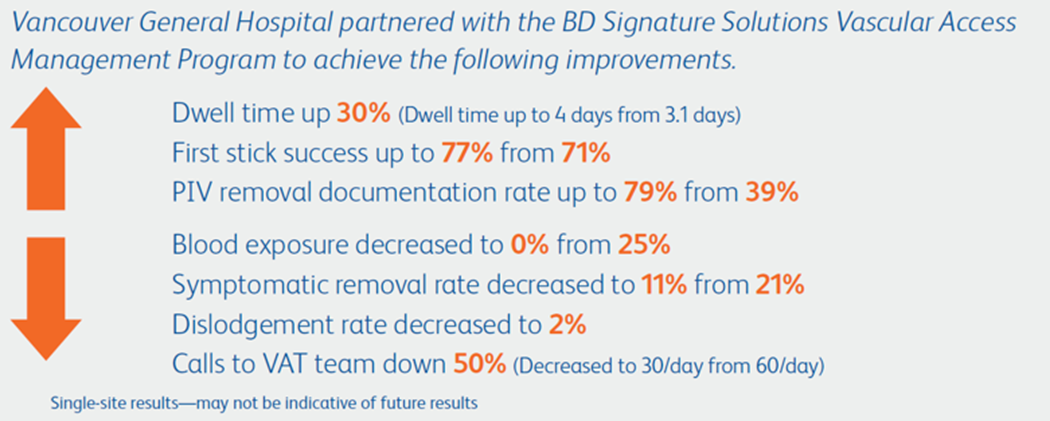
Closed system = Fully-integrated system that consists of an integrated extension tube, stabilization platform, and needle-free connectors.
*Compared to an open system.
†When used with an IV site securement dressing.
‡Compared with B. Braun Introcan Safety® catheter with Bard Statlock® IV Ultra stabilization device.
§Compared with an FEP catheter
1. González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
2. Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheterstabilization systems. J Infus Nurs. 2010;33(6):371-384.
3. Canadian Vascular Access Association. (2019). Canadian Vascular Access and Infusion Therapy Guidelines. Pembroke, ON: Pappin Communications.
4. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs. 2021;44(suppl 1):S1-S22
5. van Loon FH, Timmerman R, den Brok GP, Korsten EH, Dierick-van Daele AT, Bouwman AR. The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: Block-randomized trial. The Journal of Vascular Access. 2022;23(2):295-303.
6. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114(10):845-85