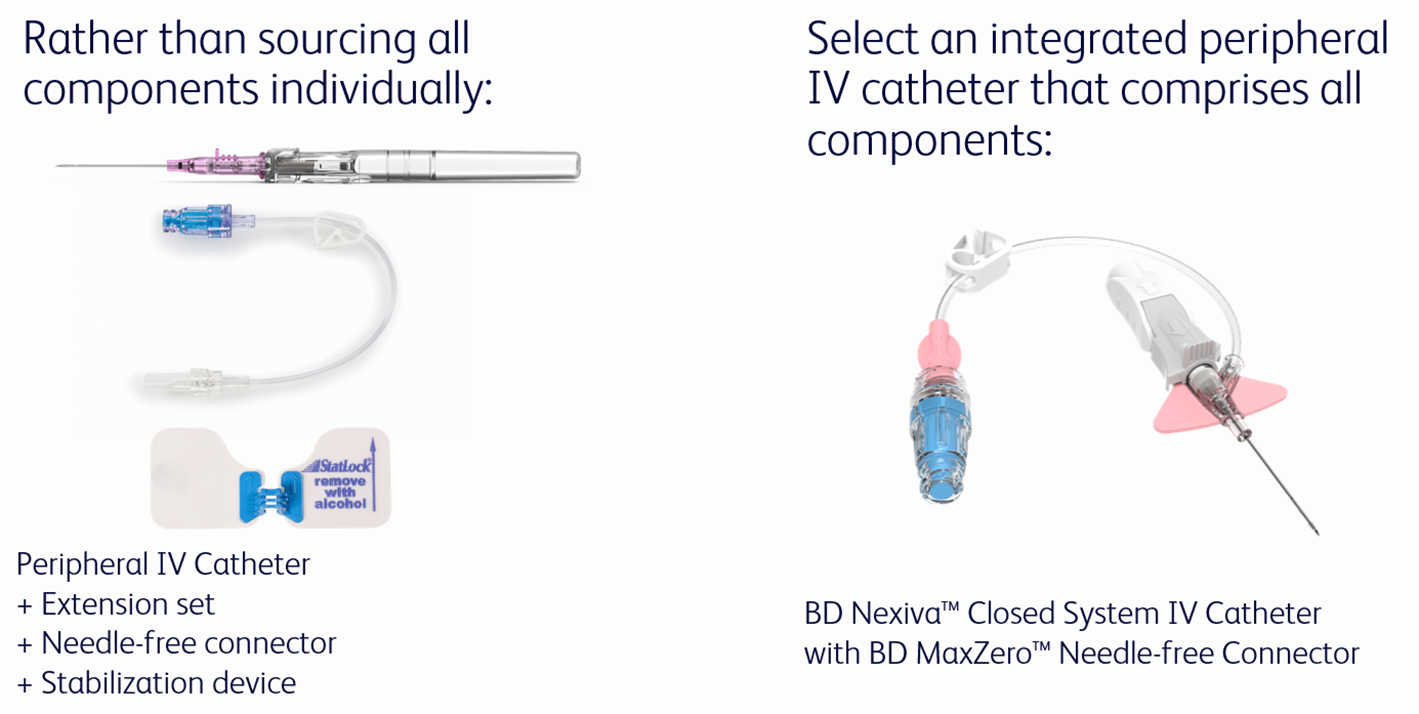
Discover how an Integrated Peripheral IV Catheter can save time (and money!)
BD Nexiva™ Closed IV Catheter System. Learn More>>

The BD Nexiva™ Closed Peripheral IV Catheter System’s unique design can help it to last longer.
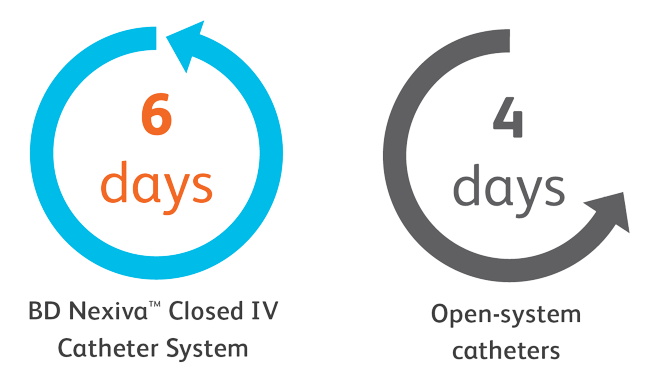
A longer dwell time, paired with clinically indicated replacement of PIVCs, can result in:
- A reduction of total IV equipment costs by up to 11%5
- Significant reduction in healthcare resource use such as equipment and staff time5
- Minimized number of restarts and costs6
- Increased patient satisfaction6
Interested in learning more?
The cost of peripheral IV catheters (PIVCs) on your healthcare organization goes beyond the price of the device
Selecting an IV catheter that can reduce the risk of complications could help you to reduce the economic burden of complications in your organization.
Learn how BD Nexiva™ can make your IV practice safer for yourself and your patients
Discover the difference of a closed PIVC system.
Preserve sites and protect veins
Each time the PIVC is manipulated there is a risk of blood exposure and a greater chance of patient complications. Learn more about the various Vascular Access Device (VAD) guidelines and standards of practice.

Fortunately, there are many guidelines and best practices that can help to elevate the standard of care you provide, which can have wider-reaching benefits to your organization. Learn how the BD Nexiva™ Closed Peripheral IV Catheter System aligns with the Canadian Vascular Access & Infusion Therapy Guidelines7. The goal is to have the Peripheral IV Catheter last the length of therapy, without complications to the patient, while also safeguarding the healthcare worker who is performing the insertion.
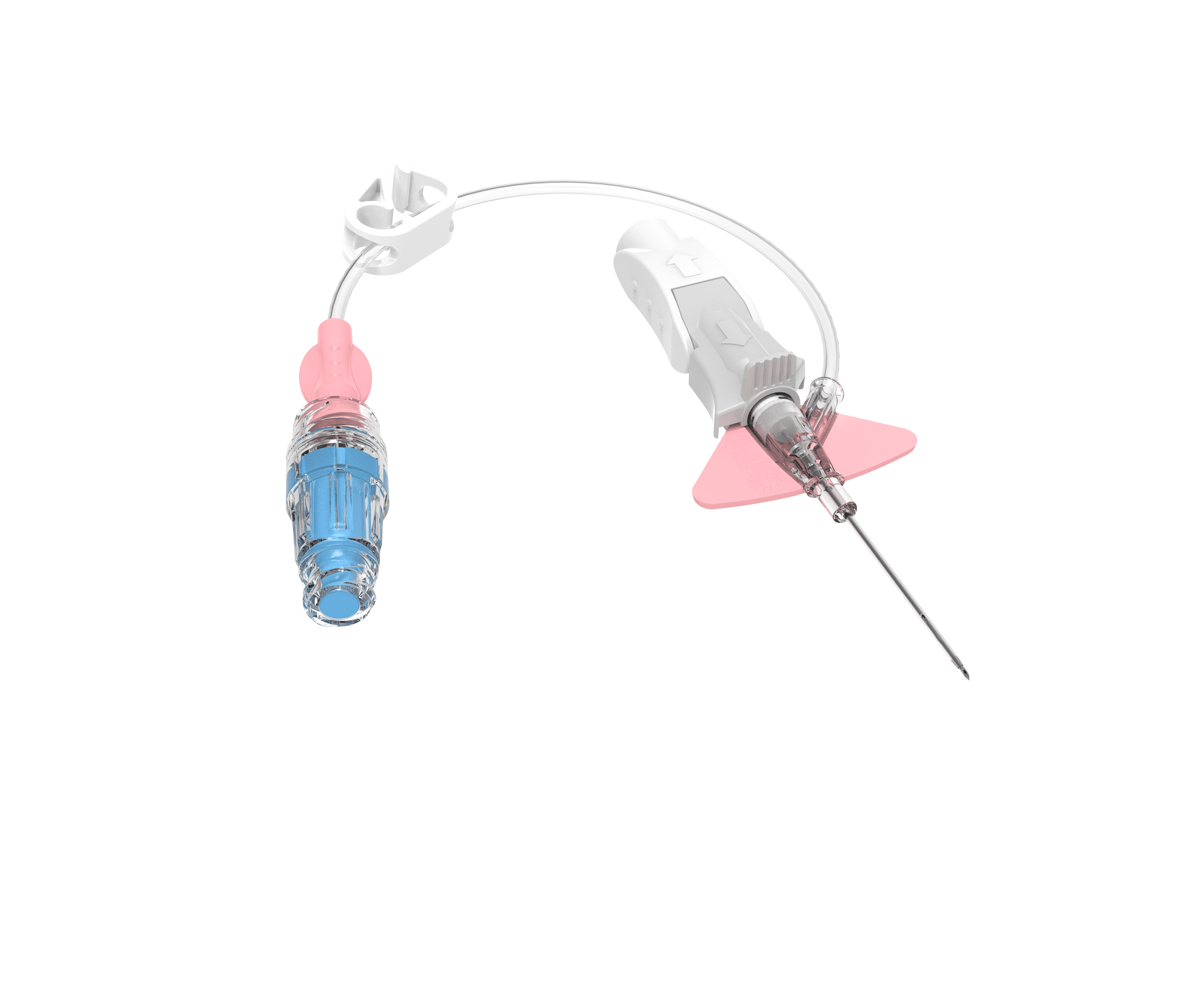
Integrated Extension Set
Use extension set (integrated preferred) on Peripheral Vascular Access Device (PVAD) (to reduce manipulations and avoid dislodgement).
Passive Safety Needle-Shielding Mechanism
Use safety-engineered medical sharps.
Built-in Stabilization Platform
Use suture-less securement (including engineered securement devices) to limit movement of VAD. Method of securement can include an integrated stabilization feature on PVAD
Do you have a clinical colleague who is interested in learning more about best practice guidelines and standards?
Click here: https://go.bd.com/Nexiva-safety.html

Each department can have different clinical needs and components may need to be discarded and added throughout the journey, adding to your costs. Each time the PIVC is manipulated there is a risk of blood exposure and a greater chance of patient complications. This is the reality when you use a catheter that is NOT integrated.
Compliance with best practices around PIVC use may help reduce costs, limit resource utilization, and increase patient satisfaction. 2-6
REFERENCES
2. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs. 2021;44(suppl 1):S1-S22
3. O’Grady NP, Alexander, M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16
4. Alexander M, Corrigan A, Gorski L, et al. Infusion Nursing: An Evidence-Based Approach. 3rd ed. St. Louis, MO: Saunders Elsevier; 2010:213, 410.
5. Tuffaha H, Rickard C, Scuffham P, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58.
6. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-107
7. Canadian Vascular Access Association. (2019). Canadian Vascular Access and Infusion Therapy Guidelines. Pembroke, ON: Pappin Communications.
8. González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
9. Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheterstabilization systems. J Infus Nurs. 2010;33(6):371-384.
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.