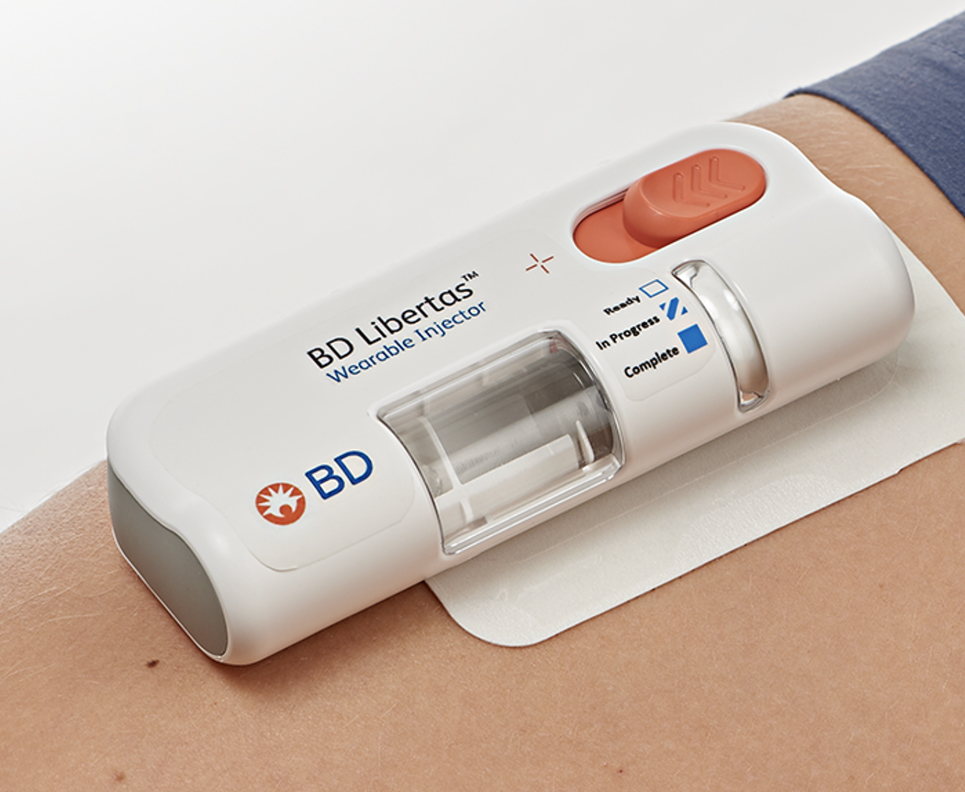
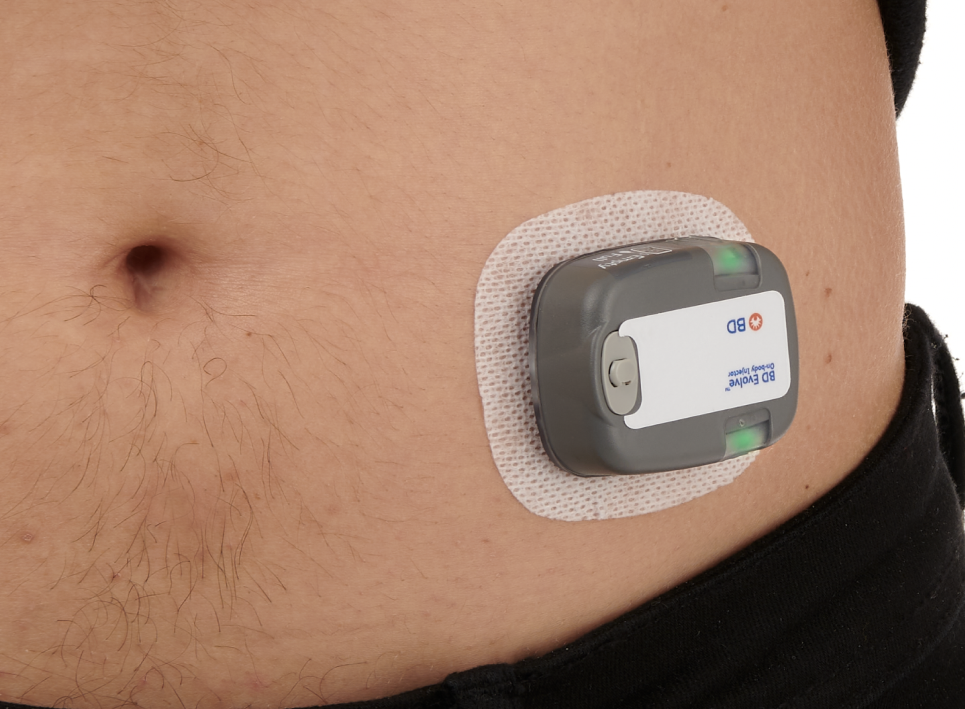
Combination product development solutions for subcutaneous drug delivery
There are many risk factors to consider when developing combination products.
Our wearable solutions are evaluated and tested, with multiple pre-clinical, clinical and human factors studies conducted to inform device design and evaluate injection performance. We have robust data that supports a variety of combination product development needs and configurable device components to satisfy final drug formulation and delivery time objectives.
BD is your experienced partner, here to help de-risk your product development and commercialization journey. We offer broad support and a flexible portfolio of wearable drug delivery solutions that integrate with established drug filling technologies.