Get specific, actionable insights on an extended set of HPV genotypes
Discovery. Diagnostics. Delivery.
Can be accessed on our Customer Learning Portal. The e-learning courses offer detailed and solution focused training to help better understand how to solve for common scenarios.
Discovery. Diagnostics. Delivery.
Experts from around the globe describe the intersection between patient and healthcare worker safety, focusing on techniques to improve both occupational and public health.
Webinar Objectives:
- Describe the current global impact of bloodborne disease
- Describe occupational exposure incidents for needlesticks and sharps injuries
- Define safety as a function of focus for both patient and health worker, to reduce overall bloodborne disease
- Provide guidance on the importance of prevention programs including use of safer medical devices, immunization/vaccination programs, and safe clinical practices
- Illustrate an effective pathway for reporting exposure incidents and injuries
- Define processes for post-exposure medical treatment and prophylaxis
- Share global experiences from key stakeholders responsible for sharps safety and public health programs around the world
Save Your Seat
BD Onclarity™ HPV Assay: the only FDA-approved HPV test
that individually identifies HPV 31
The BD Onclarity™ HPV Assay with extended genotyping allows for a more precise, accurate way to measure a woman’s risk for developing cervical pre-cancer and cancer compared to an assay with partial genotyping.2,5-9

Consensus guidelines for management of abnormal cervical cancer screening results and changes in HPV genotype prevalence are impacting clinical management and calling for a shift towards next-generation HPV screening with extended genotyping.1-4

Cervical cancer screening & management guidelines favor a personalized risk-based management with HPV testing as the foundation for risk-estimation.1
| Learn more |
As the vaccinated population increases, HPV 16 and 18 are decreasing in prevalence, making it crucial to identify the other high-risk HPV genotypes.3,4
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
There are two types of HPV assays - those with partial genotyping and those with extended genotyping2,10

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
HPV assays with partial genotyping
- Report multiple high-risk HPV genotypes in a single, pooled result10
- May mask the true risk of CIN3+ disease due to HPV 31 and will likely lead to a one-year follow-up recommendation instead of an immediate colposcopy referral7-9
- Prohibit monitoring of genotype-specific high-risk HPV persistence beyond HPV 16 and 182
BD Onclarity™ HPV Assay, the only FDA-approved assay with extended genotyping
- Reports 6 high-risk HPV genotypes individually and the other 8 high-risk genotypes in strategic groupings10,11
- Can individually identify HPV 31, which poses a similar risk for cervical pre-cancer and cancer as compared to HPV 182
- Can track genotype-specific high-risk HPV persistence – the most important determinant of cervical cancer risk in women who test HPV-positive, regardless of HPV genotype2,7-9
| Learn more |
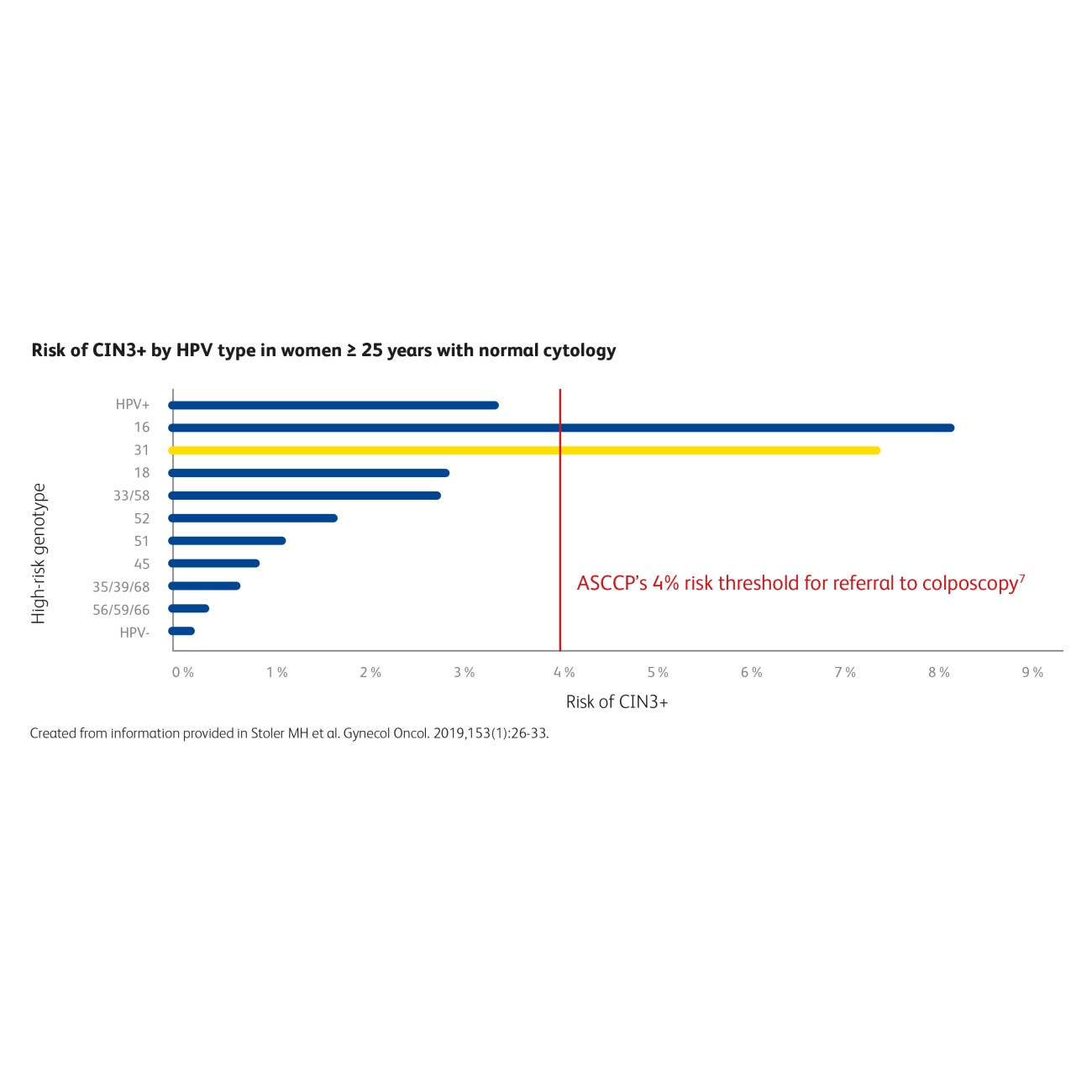
HPV 31 identification matters.
Extended genotyping is critical.
Following the ASCCP principle of “similar management for similar risk”, women with an immediate risk for CIN3+ disease above 4% should be referred to colposcopy.1 Women 25 years and older with HPV 31 and normal cytology had an immediate risk for CIN3+ of 7.5%, similarly to HPV 16, with a risk of 8.1%.5
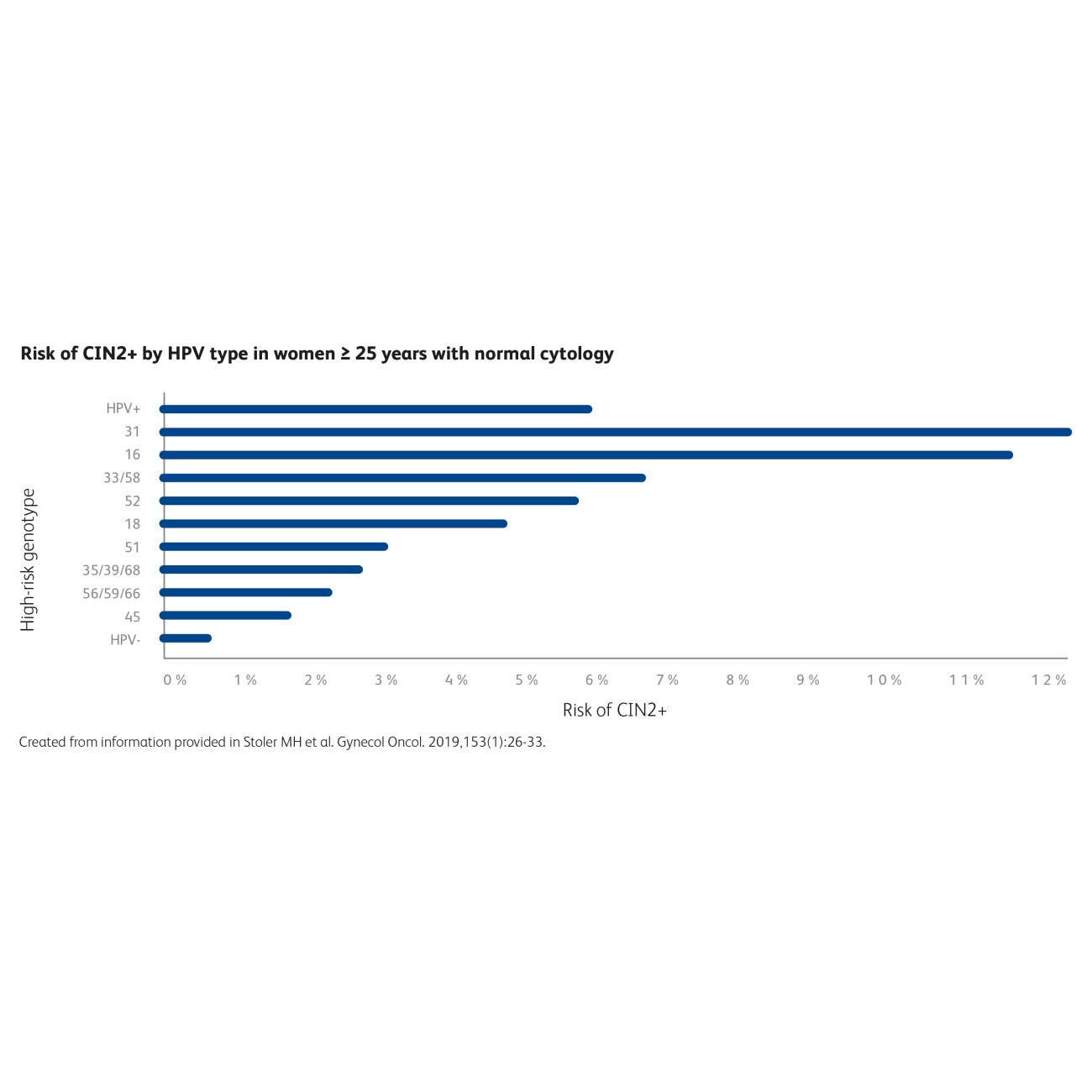
Know the HPV genotype, know the risk.
Women 25 years and older with HPV 31 and normal cytology had an immediate risk for ≥CIN2 of 12.1%, similarly to HPV 16, with a risk of 11.6%.5
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
HPV 31 identification matters. Extended genotyping is critical.
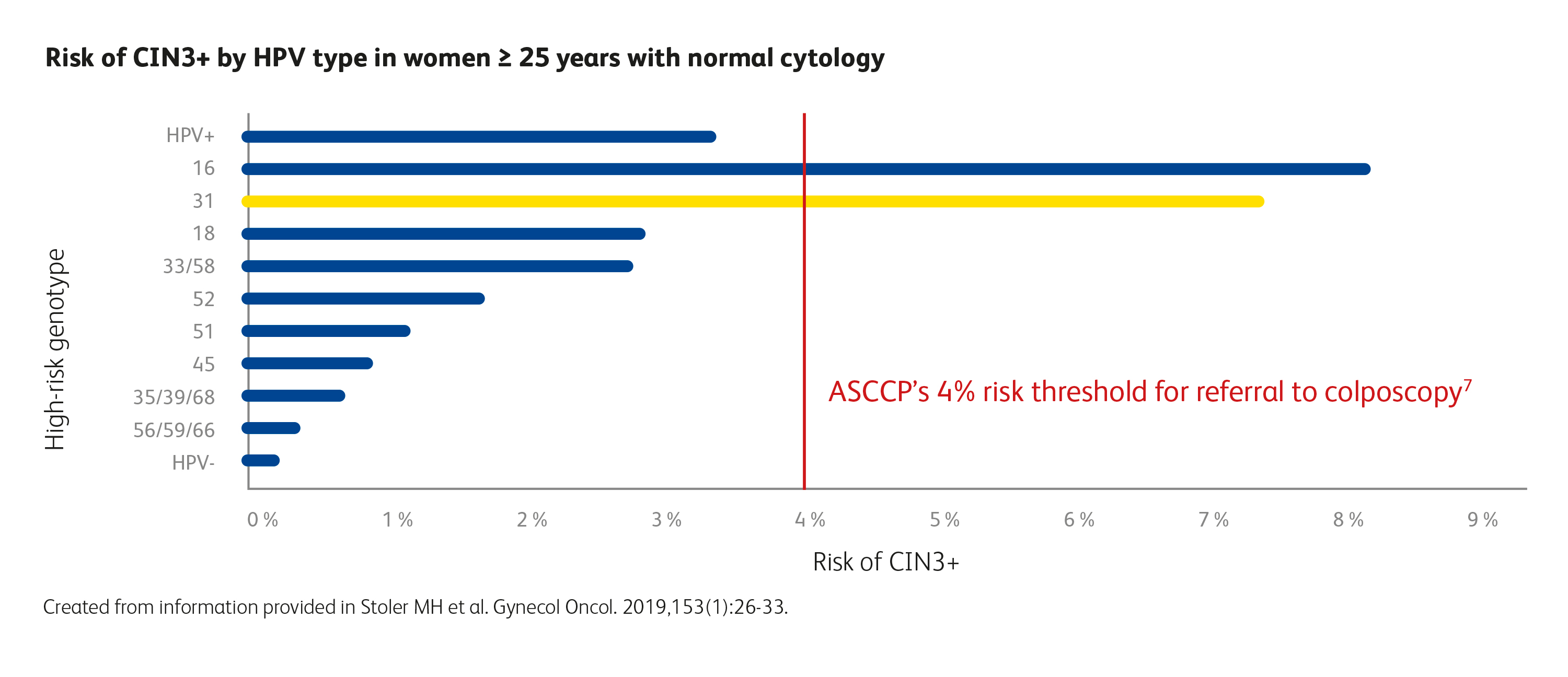 Following the ASCCP principle of “similar management for similar risk”, women with an immediate risk for CIN3+ disease above 4% should be referred to colposcopy.1 Women 25 years and older with HPV 31 and normal cytology had an immediate risk for CIN3+ of 7.5%, similarly to HPV 16, with a risk of 8.1%.5
Following the ASCCP principle of “similar management for similar risk”, women with an immediate risk for CIN3+ disease above 4% should be referred to colposcopy.1 Women 25 years and older with HPV 31 and normal cytology had an immediate risk for CIN3+ of 7.5%, similarly to HPV 16, with a risk of 8.1%.5
Know the HPV genotype, know the risk.
Women 25 years and older with HPV 31 and normal cytology had an immediate risk for ≥CIN2 of 12.1%, similarly to HPV 16, with a risk of 11.6%.5
| Learn more |
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Learn more about extended genotyping with the BD Onclarity™ HPV Assay
REFERENCES
1. Perkins RB et al. J Low Genit Tract Dis. 2020;24:102-131. 2. Bonde JH et al. J Low Genit Tract Dis. 2020;24(1):1-13. 3. Wright TC et al. Gynecol Oncol. 2019;153(2):259-265. 4. Drolet M et al. Lancet. 2019;394(10197):497-509. 5. Stoler MH et al. Gynecol Oncol. 2019;153(1):26-33. 6. Bonde J et al. Int J Cancer. 2019;145:1033-1041. 7. Elfgren K et al. AM J Obstet Gynecol. 2017;216(3):264e1-264.e7. 8. Radley D et al. Hum Vaccin Immunother. 2016;12(3):768-772. 9. Bodily J, Laimins LA. Trends Microbiol. 2011;19(1):33-39. 10. Salazar K et al. J Am Soc Cytopath. 2019;8:284-292. 11. BD Onclarity HPV Assay US Package Insert [8089894].
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.


