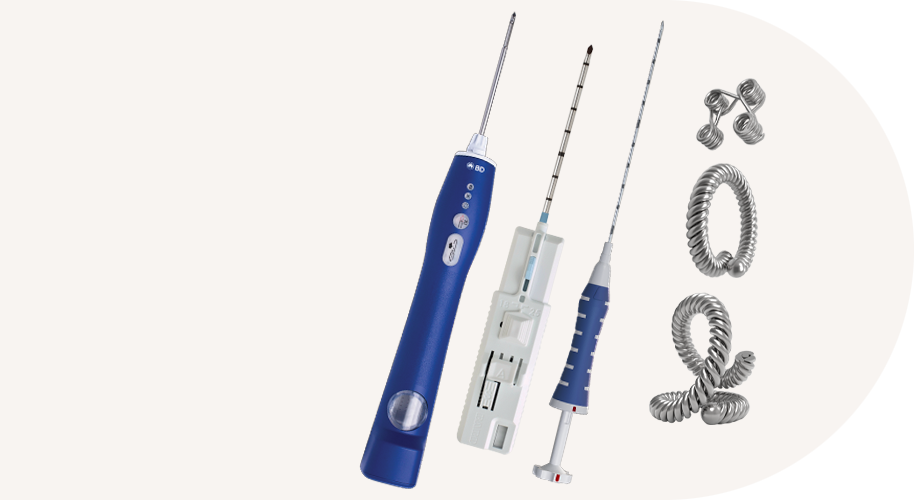
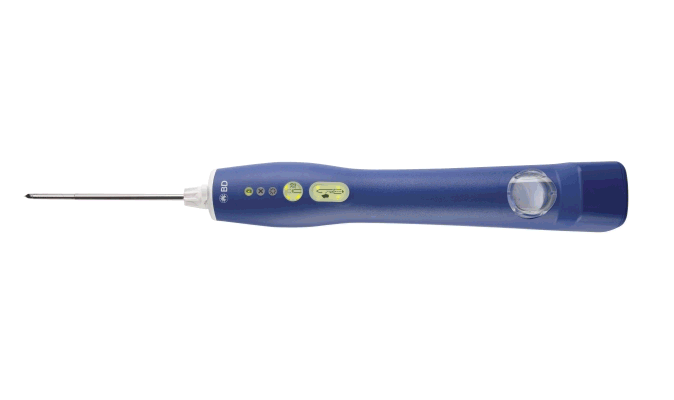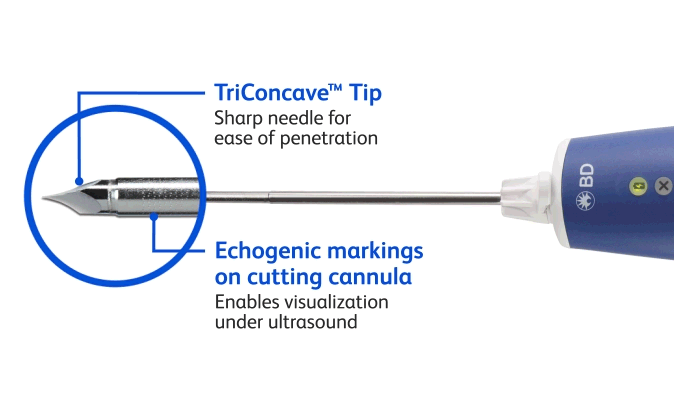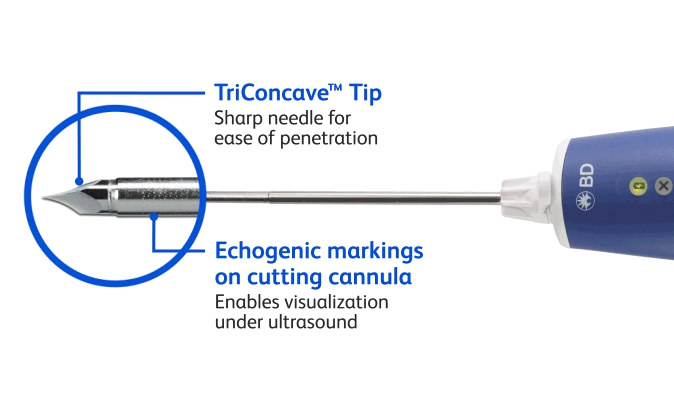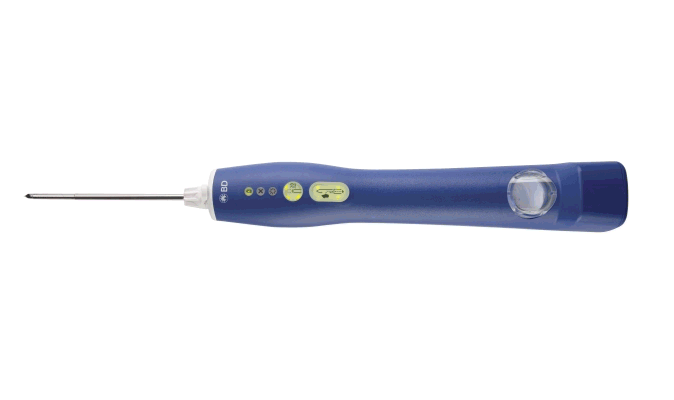
BD EleVation™ Breast Biopsy System (For information on UltraCor™ Twirl™ Breast Tissue Marker, please continue scrolling.)
Indications for Use:
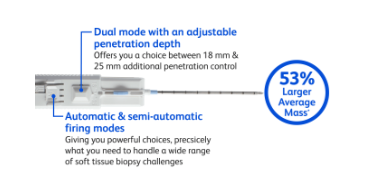
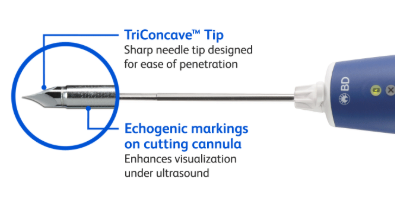
The BD EleVation™ Breast Biopsy System is indicated to obtain tissue samples from the breast or axillary lymph nodes for diagnostic analysis of breast abnormalities. The BD EleVation™ Breast Biopsy System is intended to provide breast tissue for histologic examination with partial or complete removal of the imaged abnormality. The extent of histologic abnormality cannot be reliably determined from its mammographic appearance.
Therefore, the extent of removal of the imaged evidence of an abnormality does not predict the extent of removal of a histologic abnormality, e.g. malignancy. When the sampled abnormality is not histologically benign, it is essential that the tissue margins be examined for completeness of removal using standard surgical procedures.
Contraindications:
1. The BD EleVation™ Breast Biopsy System is for diagnostic use only, NOT for therapeutic use.
2. The BD EleVation™ Breast Biopsy System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings:
1. Patients who may have a bleeding disorder, or who are receiving anticoagulant therapy, may be at increased risk of complications.
2. As with any biopsy instrument, there is a potential risk for infection.
3. The BD EleVation™ Breast Biopsy System should not be used in a Magnetic Resonance Imaging (MRI) Suite.
4. The BD EleVation™ Breast Biopsy System has not been tested using stereotactic guidance or for use
with an MRI.
5. The BD EleVation™ Breast Biopsy System should not be used in an operating room.
6. The BD EleVation™ Breast Biopsy System is not classified as an AP or APG device.
7. The BD EleVation™ Breast Biopsy System is not suitable for use in the presence of flammable anesthetic.
8. The BD EleVation™ Breast Biopsy System is not suitable for use in an oxygen rich environment.
9. The BD EleVation™ Driver must only be used with BD EleVation™ Probes and BD EleVation™ Accessories.
10. All breast biopsies should be performed under ultrasound guidance to confirm the BD EleVation™ Probe’s position relative to the target region to be sampled and to help mitigate the occurrence of a false negative biopsy. The BD EleVation™ Breast Biopsy System is intended for use with ultrasound imaging only.
11. The battery may only be replaced or disposed of by an authorized Service and Repair facility.
12. Use only with supplied AC power BD EleVation™ Accessories. Removing the AC adapter plug from wall power shall serve as isolation means. Do not position the AC adapter plug and wireless charging stand such that it is difficult to remove the AC adapter plug from the wall outlet if needed to remove mains power.
13. Do not reuse BD EleVation™ Probe. Reusing the BD EleVation™ Probe bears the risk of cross-patient contamination as biopsy probes, particularly those with long and small lumina, joints, and/or crevices between components, are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the BD EleVation™ Probe for an
indeterminable period of time. The residue of biological material can promote the contamination of the BD EleVation™ Probe with pyrogens or microorganisms which may lead to infectious complications.
14. Do not resterilize BD EleVation™ Probe. After resterilization, the sterility of the BD EleVation™ Probe is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilizing the BD EleVation™ Probe increases the probability that it will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
Precautions:
1. The BD EleVation™ Breast Biopsy System should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques.
2. Do not attempt to remove the cover or modify the device in any way.
Potential Complications:
1. Potential complications are those associated with any percutaneous removal/biopsy technique for tissue collection. Potential complications are limited to the region surrounding the biopsy site and include hematoma, lymphedema, hemorrhage, infection, non-healing wound, pain, nerve injury, and tissue adherence to the BD EleVation™ Probe while removing it from the breast.
2. As per routine biopsy procedures, it may be necessary to cut tissue adhering to the BD EleVation™ Probe while removing it from the breast.
Please consult package insert for more detailed safety information and instructions for use.
BD and the BD logo, and BD EleVation Breast Biopsy System are trademarks of Becton, Dickinson and Company or its affiliates. ©2024 BD. All rights reserved.
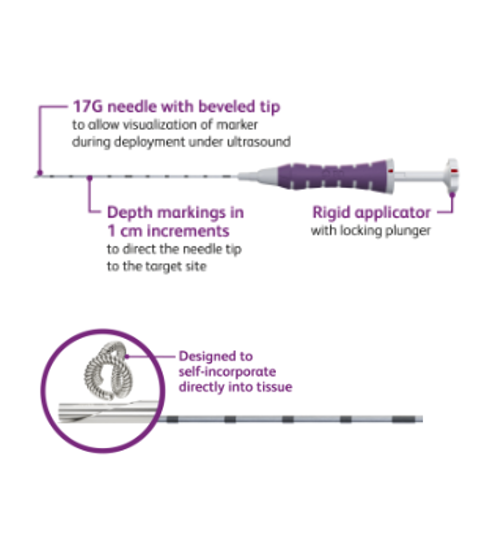
UltraCor™ Twirl™ Breast Tissue Marker
Indications for Use: The UltraCor™ Twirl™ Breast Tissue Marker is intended for use to attach to soft breast tissue, including axillary lymph nodes, to radiographically mark the location of the biopsy procedure.
Contraindications: Patients with a known hypersensitivity to the materials listed in the device description may suffer an allergic reaction to this implant. The implant is made from a nickel-titanium alloy; if there is a known allergy to nickel, use of the UltraCor™ Twirl™ Breast Tissue Marker is not advised. This device is not intended for use except as indicated above.
Warnings:
• As with any foreign object implanted into the body, potential adverse reactions are possible. It is the responsibility of the physician to evaluate the risk/benefit prior to the use of this device.
• Use caution when inserting near a breast implant to avoid puncture of the implant capsule.
• This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable period of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications. Additionally, re-use and/or repackaging may compromise the structural integrity and/or material and design characteristics of the device, which may lead to device failure, and/or lead to patient injury.
• Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
• After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and local, state and federal laws and regulations. Dispose of sharps in designated sharps disposal containers. This product should be disposed of appropriately, not recapped or resterilized.
• Keep dry. Keep away from sunlight.
• Examine the product to ensure it has not been damaged during shipment and that its size, shape, and condition are suitable for the procedure for which it is to be used. Do not use if product damage or contamination is evident.
Precautions:
• Do not use if needle is bent and/or tip is damaged.
• Use caution when handling the device to prevent premature deployment of the breast tissue marker.
Potential Complications: Complications may occur at any time during or after the procedure. Potential complications of breast tissue marker placement may include, but are not limited to: hematoma/bleeding, hemorrhage, infection, lymphedema, unspecified tissue injury, pain, marker migration, allergic reaction and inaccurate marker placement, which may affect the accuracy of future tissue diagnoses.
Note: Users should report any serious incident that has occurred in relation to the device to the manufacturer and the regulatory authority of the country in which the user and/or patient is established.
Please consult packaging inserts for more detailed safety information and instructions for use.
BD and the BD logo, Twirl and UltraCor are trademarks of Becton, Dickinson and Company or its affiliates. ©2025 BD. All rights reserved.