BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.
Contact us | unsubscribe | My profile
* Based on BD experience working with customers.
# When compared to the BD SCF™ FluroTec® ∞ Plunger Stopper.
** Results based on a sample of 100 pieces of BD Flurotec® and BD SCF™ PremiumCoat®. Variables compared were Mean (glide force reduction) and standard deviation (glide force variability).
^ Injection time is defined as the time required from the start of the injection (start of stopper translation) to the time required to empty the reservoir or prefillable syringe. Injection time was measured in air with a PEG (polyethylene glycol) filled syringe. Injection time variability refers to consistency of results (or reduced dispersion) for injection time with the BD SCF™ PremiumCoat® Plunger Stopper, BD Neopak™ Glass Prefillable Syringe and 2-steps BD Disposable Autoinjector compared to Flurotec® 1mlL. Injection time was measured in air through benchtop testing with the BD SCF™ PremiumCoat® Plunger Stopper, BD Neopak™ Glass Prefillable Syringe and 2-steps BD Disposable Autoinjector.
^^ No “Ribs not touching after irradiation” criteria in the PremiumCoat® customer specification: Becton Dickinson and Company. BD SCF™ PremiumCoat® Plunger Stopper 1-3mL Customer quality specification; Results based on a sample of 100 pieces of BD Flurotec® and BD SCF™ PremiumCoat®.
† Extractables analysis with glass cane and by immersion showed that the coating provides a barrier effect for a number of potential extractables in the 6720 formulation, for elemental impurities and semi-volatile and non-volatile organic molecules. Alternative stopper tested is FluroTec® 1mlL.
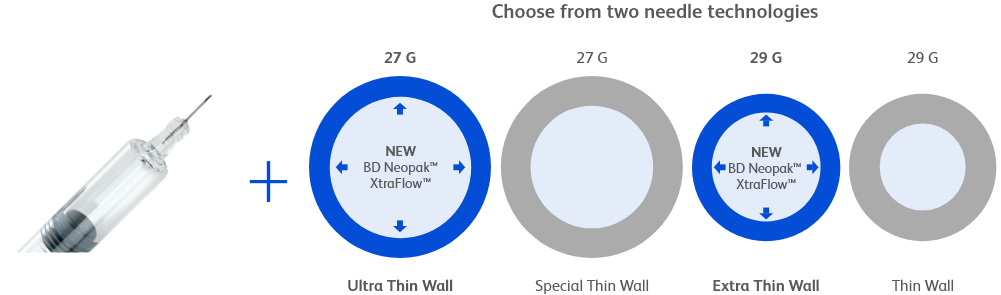
‡ For a 30cP solution. With BD Neopak™ XtraFlow™ 27G 8mm ultra-thin wall syringe when compared to a 12.7mm 27G special thin-wall syringe. Ejection force and injection time values were simulated through a mathematical model based on the Hagen-Poiseulle equation. For injection time reduction, a constant force was defined. For injection force reduction, a fixed time was defined.
µ With BD Neopak™ XtraFlow™ 27G 8mm ultra-thin wall syringe when compared to a 12.7mm 27G special thin-wall syringe.
PDA: Parenteral Drug Association; PMF: Public Master File; FDA: U.S. Food & Drug Administration; USP: U.S. Pharmacopoeia; EMA: European Medicines Agency; PFS: Prefillable Syringes; ETFE: Ethylene tetrafluoroethylene.
References:
1. Food and Drug Administration. 2024 Recalls. FDA website. Accessed on April 2, 2024, at https://datadashboard.fda.gov/ora/cd/recalls.htm.
2. Pager A, Duinat B, Alves B. How Shorter Needles with Thinner Walls are Set to Improve the Injection Experience in Chronic Care. ONdrugDelivery Magazine. 2020; 107: 37-42. https://ondrugdelivery.com/how-shorter-needles-with-thinner-walls-are-set-to-improve-the-injection-experience-in-chronic-care/
3. Injection time and ejection force calculation [internal study], Le Pont-de-Claix, France; Becton, Dickinson and Company; 2021.
4. Cilurzo F, Selmin F, Minghetti P, et al. Injectability evaluation: an open issue [published correction appears in AAPS PharmSciTech. 2016 Dec;17 (6):1508]. AAPS PharmSciTech. 2011;12(2):604-609. doi:10.1208/s12249-011-9625-y
5. GMP-Compliance. 2018 European Pharmacopoeia: New Chapter on Visual Inspection for Visible Particles. GMP Compliance website. https://www.gmp-compliance.org/gmp-news/european-pharmacopoeia-new-chapter-on-visual-inspection-for-visible-particles. Accessed April 2, 2024.
6. Johns J, Golfetto P, Bush T, et al. Achieving “Zero” Defects for Visible Particles in Injectables. PDA J Pharm Sci Technol. 2018;72(6):640-650. doi:10.5731/pdajpst.2018.009027
7. U.S. Food and Drug Administration. 2024 SOPP 8415: Procedures for Developing Post marketing Requirements and Commitments. FDA website. Accessed on April 18, 2024, at https://www.fda.gov/media/90591/download.
8. BDM-PS Financial file November 2020, “Actual FY20” Perimeter.
9. BD Customers compared to Evaluate Pharma ranking FY2019.
10. BDM-PS supply chain demand (BDMPS Supply Chain demand file_Desy 2018-2023), August 2021.
11. BDM-PS sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2020.
12. Registration status of drugs in BD Neopak™ Glass Prefillable Syringe, May 31st, 2024 [Internal regulatory report]. Pont-de-Claix, FR: Becton Dickinson and Company; 2024.
13. Design Control Evidence BD SCF PremiumCoat 1-3mL Plunger Stopper.
14. Becton, Dickinson and Company. Bausch + Ströbel SCF PremiumCoat® Plunger Stopper: Proof of processability. BD Medical – Pharmaceutical Systems; 2020 V-630076 2020.
15. Becton, Dickinson and Company. DVTR20234262_DV data BD SCF PremiumCoat® 1-3 mL R&D data [internal study]. Le Pont-de-Claix, France; Becton, Dickinson and Company; 2023.
16. Becton, Dickinson and Company. TR20234488 Le Pont-de-Claix, France; Becton, Dickinson and Company; 2024.
17. Becton, Dickinson and Company. DVTR20192507_DV data BD SCF PremiumCoat® 1 mlL R&D data [internal study]. Le Pont-de-Claix, France; Becton, Dickinson and Company; 2023.
18. Becton, Dickinson and Company. TR20233592 – Extractables data on BD PremiumCoat® 1-3 mL coated and non-coated Stoppers.
19. BD SCF™ PremiumCoat® Plunger Stopper 1-3mL Customer Quality Specification; 2023.
20. Becton, Dickinson and Company. Prefilled Syringe Customer Requirements Research, Voice of the Customer [internal study]. Le Pont-de-Claix, France; Becton, Dickinson and Company; 2018.
21. Becton, Dickinson and Company. BD SCF™ PremiumCoat® 1mlL Plunger stopper simulated extractable and leachable study [internal study]. Pont-de-Claix, FR: Becton Dickinson and Company; 2019.
22. BD Neopak™ XtraFlow™ 2.25 mL prototype evaluation [internal study] , Le Pont-de-Claix, France; Becton, Dickinson and Company, 2020.
23. Pager A, Combedazou A, Guerrero K, et al. User experience for manual injection of 2 mL viscous solutions is enhanced by a new prefillable syringe with a staked 8 mm ultra-thin wall needle. Expert Opin Drug Deliv. 2020;17(10):1485-1498. doi:10.1080/17425247.2020.1796630
24. Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519-1530. doi:10.1185/03007995.2010.481203