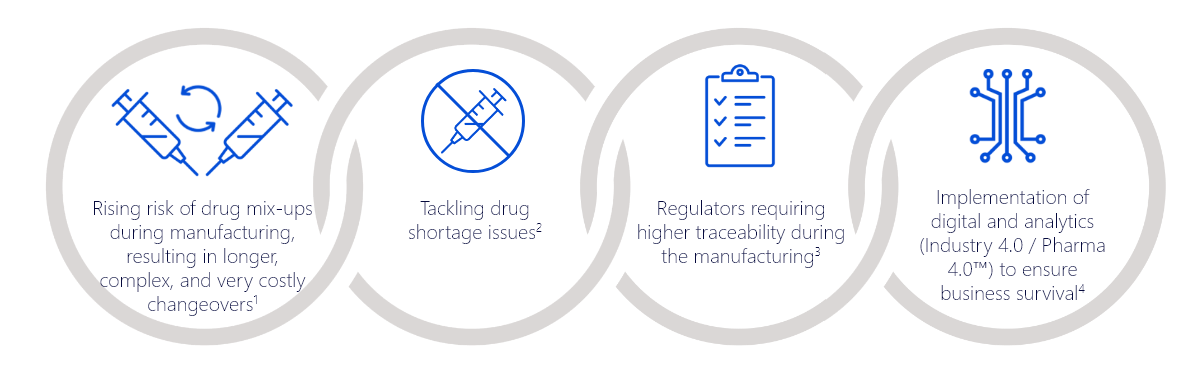
Primary Challenges Around Aseptic Filling that Traceability of Primary Containers can Help Address:

Agility Starts With Digital Visibility

Unit-level digital visibility of the primary container is the lifeblood of agile fill/finish operations.
Ready-to-fill syringes will incorporate a Container Unique Identifier (CUID) using Radio Frequency Identification (RFID) technology.
At the end of the BD manufacturing process, tagged syringes are placed into tubs and their CUIDs are read and aggregated to the tub/nest Identifier (ID).
This aggregation of parent and child units ensures tag readability prior to delivery to the customer filling line, establishing both data integrity and syringe pedigree.
When the syringes are loaded onto pharma filling lines, one reading of the tub/nest ID is enough to associate it with its current drug filling batch. Therefore, each unique syringe ID is associated to the product filling batch and drug code.
At each subsequent process step, the syringe ID can be cross-checked to prevent mix-ups and additional manufacturing events are expected to be recorded to build a full syringe pedigree.
Prior to release, the drug’s pedigree can be confirmed based on its movement through the pharmaceutical manufacturing steps.

Let's have a conversation


Traceability enables unit-level visibility with the potential to log the container’s passage through each manufacturing step, creating a chain of custody record.
Ready-to-fill syringes will incorporate a unique identifier (UID) using radio frequency identification (RFID) technology.
At the end of the BD manufacturing process, tagged syringes are placed into tubs and their UIDs are read and aggregated to the tub/nest ID.
This aggregation of parent and child units ensures tag readability prior to delivery to the customer filling line, establishing both data integrity and syringe pedigree.
When the syringes are loaded onto pharma filling lines, one reading of the tub/nest ID is enough to associate it with its current drug filling batch. Therefore, each unique syringe ID is associated to the product filling batch and drug code.
At each subsequent process step, the syringe ID can be cross-checked to prevent mix-ups and additional manufacturing events are expected to be recorded to build a full syringe pedigree.
Prior to release, the drug’s pedigree can be confirmed based on its movement through the pharmaceutical manufacturing steps.

Drug mix-ups are costly in terms of staff time and lost product:1
Colour-coded product identification is limited by the camera’s ability to distinguish between similar colours.1
BD syringes, pre-tagged with serial numbers associated with the drug filling batch, are designed to ensure product identification throughout the manufacturing process, helping to prevent mix-ups.
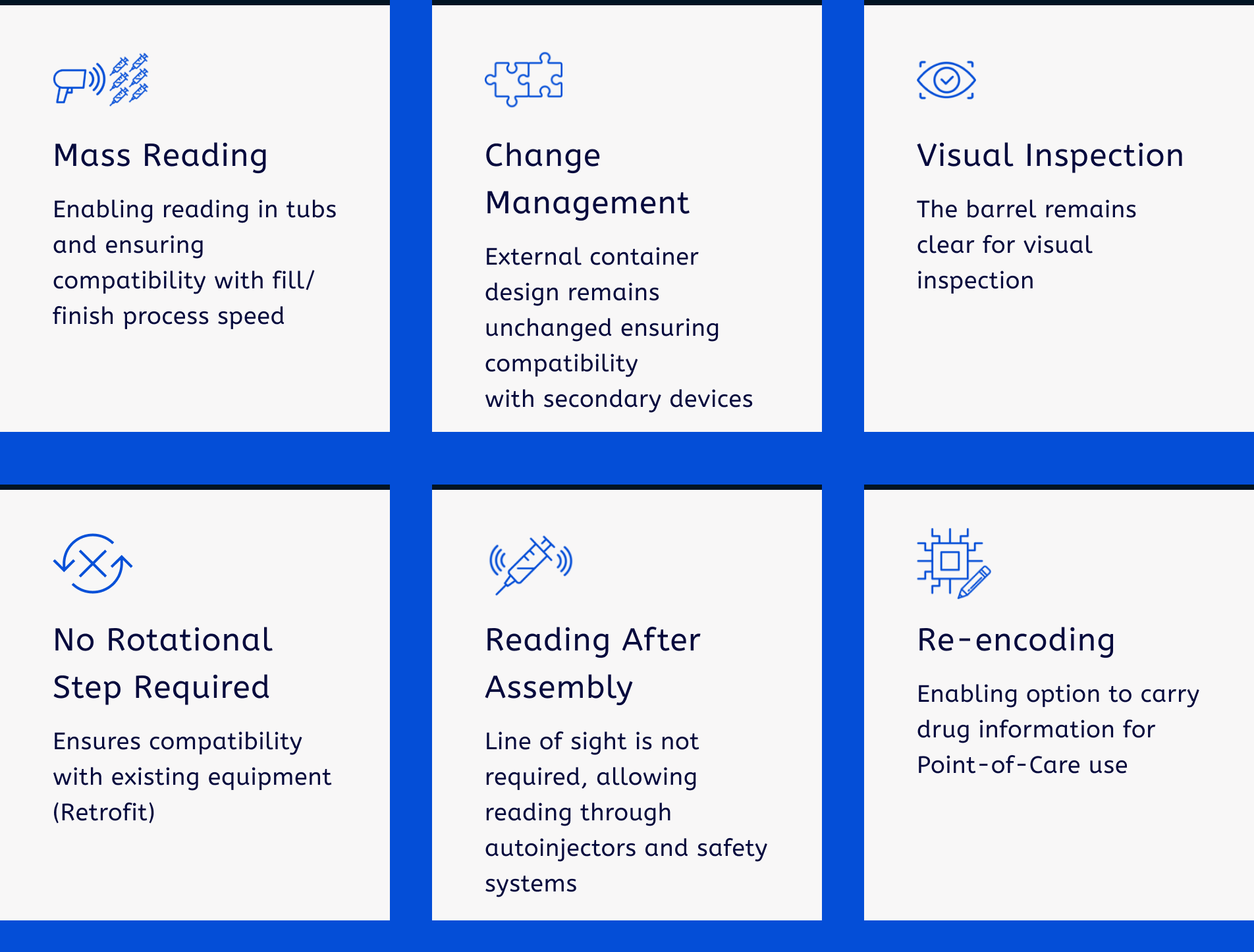
BD iDFill™ Individual Prefillable Syringe Identification offers an optimal balance between the high potential of use cases and the cost of implementation.
BD iDFill™ Individual Prefillable Syringe Identification aims to give you:
| Check out this article to learn more |
Let's have a conversation
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.