Made for What’s Next in Drug Delivery
Enabling subcutaneous delivery of large volume, high viscosity biologics
SubTitle
Main Title
Sub Line
Uscid est ad quis et lam sitatium commoloribus solupta illaborum doloria alisci dioribus si quis porereh enianimus mollam, quo maxim quis ma senimus, alit,Deles etusapici am, inusdae commodi cupiderro doluptas acipsap elignatio omnihilla inihiliciu Denihit doluptamus ut ped ut eatinie ndandem. Quae. Olut exerios dipsum nis
In this webinar we have:
- Point 1
- Point 2
- Point 3
Ibus exped et ape
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Ibus exped et ape
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Title
Sub Title
Title #1
Bold main text
Italic sub text
Title #2
Bold main text
Italic sub text
Title #3
Bold main text
Italic sub text
Review our 6 scientific posters
October 22-23, 2019
During conference breajsn hear from our experts who created our posters
Workshops
Date
00/00/00/ 11:30am
main subtitle
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem Ipsum
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
John DOE
LoremIpsum
Institut of Lorem Ipsum
Date
00/00/00/ 11:30am
main subtitle
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem Ipsum
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Jenny DOE
LoremIpsum
Institut of Lorem Ipsum
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized
Engineered for superior performance with hyaluronic acid
Did you know that 72% of decision makers prefer glass syringes over plastic? Explore the benefits of the BD Hylok™ Glass Syringe and other unique features.3
BD Hylok™ Glass Prefillable Syringe for hyaluronic acid
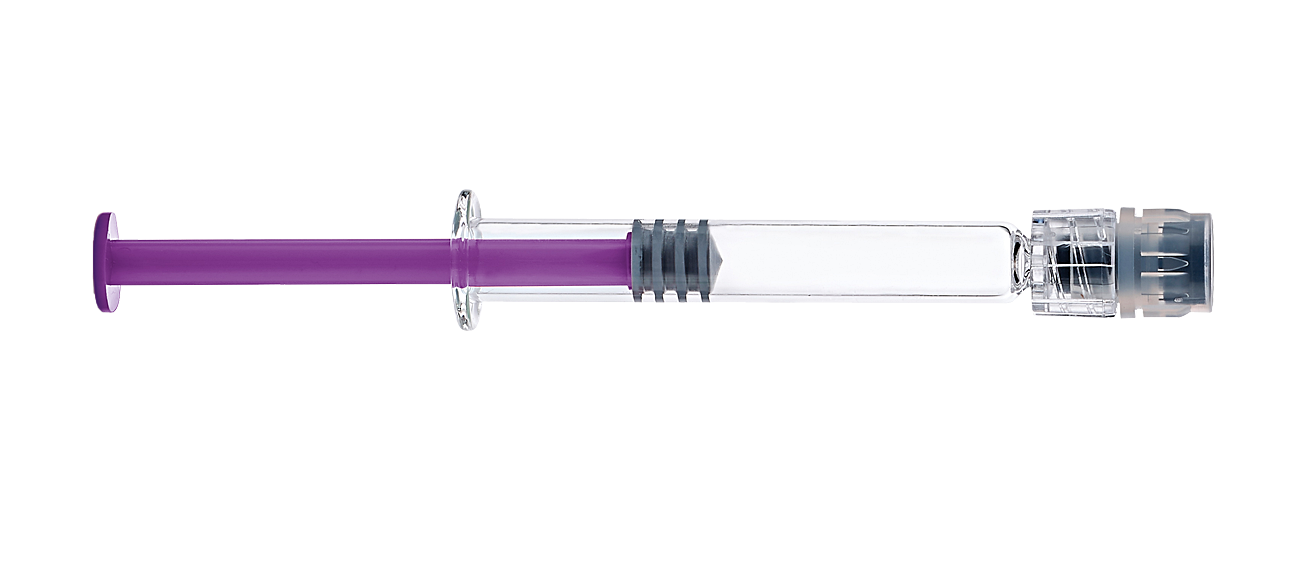
Screw on tip cap
Easy to use and intuitive tip cap with
proven user preference2
New bonding technology
Reduced risk of LLA
disconnection/rotation1,2
New LLA thread design
Safe and robust needle
connection4
Glass barrel
Limited risks and costs associated with
potential container change
Testimonials
See how devices like our BD Hylok™ Syringe are changing the way dermatologists look at glass syringe performance
Introducing the BD Libertas™ Wearable Injector
Subcutaneous Delivery of Large Volume Complex Biologics
- The BD Libertas™ Wearable Injector is designed to deliver subcutaneous injections of large volume (2-5 mL or 5-10 mL) and/or high viscosity (up to 50 cP) fixed-dose complex biologics in home or clinical settings.1
- The 2-5 mL device was evaluated in an early feasibility clinical study with a viscous placebo for its functional performance and tissue effects, pain tolerability and overall acceptability among 52 healthy participants.*2
* An early feasibility clinical study evaluated an investigational wearable injector (BD Libertas™ Wearable Injector) for functional performance, tissue effects, subject tolerability and acceptability of 5mL subcutaneous injections with an 8 cP non-Newtonian placebo solution (at 1000 s-1 at 20°C). 52 healthy adult subjects of both genders with BMI =18.5kg/m2 were divided into two age groups, 18-64 or =65 years. An even distribution per age category and gender subgroup (i.e., males, = 65 years, etc.) was attempted across the three WHO BMI categories normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (= 30.0 kg/m2) with a final distribution of three to six subjects per subgroup. Each subject received 4 randomized injections (n=208) to both the left and right abdomen and thigh, 50% (1 thigh, 1 abdomen) with a defined movement sequence during injection. Ultrasound imagery was leveraged to qualify and quantify deposition. Tissue effects and tolerability (100mm visual analogue scale, VAS) were assessed through 24 hours with corresponding acceptability questionnaires (Likert & yes/no responses) administered through 72 hours. Wearable injectors (n=205) activated, inserted the needle and delivered a confirmed 5mL ±5% volume in 5.42 minutes (SD 0.74) before automatically retracting the needle into the wearable injector interior. All wearable injectors were completely (99%) adhered or with minor (<=24%) detachment (1%) during wear and injection without functional effect. 98.6% of depots were entirely (93.2%) or predominantly (5.4%) localized within the target subcutaneous tissue. Slight to moderate wheals (63.9%) and erythema (75.1%) were observed with =50% resolution within 30-60 minutes. Transient subject pain (tolerability) peaked mid-injection (VAS mean 9.1mm, SD 13.4) and resolved rapidly within 30 minutes (mean 0.4mm, SD 2.6). Subjects found their pain at it’s peak (=90.2%) and at injection end (=94.1%) acceptable as well as injection site appearance (96.2%) and injector wear (=92.1%), size (98%) and overall adhesive removal (=98%) acceptable with 100% likely to use the injector if prescribed. Both injection sites were viable with subject preferences divided between none (46.2%), abdomen (25%) or thigh (26.9%).1
References
1. Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton Dickinson and Company; 2014 Revision: 05
2. Woodley WD, Yue W, Morel DR, Lainesse A, Pettis RJ, Bolick NG. Clinical evaluation of an investigational 5 mL wearable injector in healthy human subjects. Clin Transl Sci. 2021;14(3):859-869. doi:10.1111/cts.12946
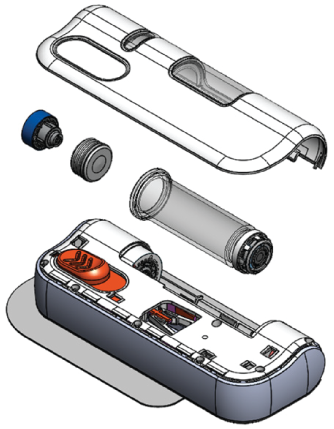
Prioritizing safety, convenience and usability throughout the design

Pre-attached adhesive pad
Transparent viewing window for drug and drug delivery visibility
Color-coded status indicator
Push button activation
- Single use, fixed dose, prefillable design
- Dose delivery options: 2-5 mL or 5-10 mL
- Mechanical spring-based power source without battery or heavy metals disposal concerns1,2
- Integrated BD Neopak™ glass primary container technology
Designed with pharma's operational processes in mind
BD Libertas™ Wearable Injector has five component modules
- Designed for pharma fill/finish utilizing standardized syringe tubs and commercial prefillable syringe filling equipment and processes1
- 100% mechanical design that does not require separate software or electronics supplier management and revision controls2
Container and device samples available upon request.
Delivering the advantage of experience
Additional BD research into large volume subcutaneous injections
Additional BD research into large volume subcutaneous injections may be found in another published clinical study in the peer reviewed journal Clinical and Translational Science.
Additional translational feasibility study to examine an expanded range of volumes and viscosities
This additional translational feasibility study examined an expanded range of volumes and viscosities (up to 10ml & 20cP) using delivery from a surrogate syringe pump and without co-delivery of a permeation enhancer in 32 healthy adult subjects.
*BD Libertas™ Wearable Injector and DCA Design International have won two design awards: the Good Design® Award and the iF Design Excellence Award.
Access the full published journal article “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects” below.
Request article access*BD LibertasTM Wearable Injector and DCA Design International have won two design awards: the Good DesignⓇ Award and the iF Design Excellence Award.
**Early feasibility clinical study of investigational BD Libertas™ Wearable Injector (WI) evaluated 5 mL, non-Newtonian ~8cP subcutaneous placebo injections in 52 healthy adult subjects of ≥18.5kg/m2 BMI divided into 2 age groups (18-64 or ≥65 years) for functionality, tissue effects, subject tolerability and acceptability.
Learn how BD supports successful commercialization of combination products by enabling the delivery of biologics.
Learn moreDelivering the advantage of experience
More than 50 pre-clinical3 and clinical studies4
BD has conducted over 50 BD pre-clinical3 and clinical4 studies to inform the design and development of the BD Libertas™ Wearable Injector.
High device acceptance
BD reinforced its commitment to innovation by completing a 52-subject human trial for its award-winning 2-5 mL BD LibertasTM Wearable Injector.5 100% of study subjects likely to use if prescribed.5** Results of the human clinical trial with the 2-5 mL BD LibertasTM Wearable Injector are published in Clinical and Translational Science.5
*BD LibertasTM Wearable Injector and DCA Design International have won two design awards: the Good DesignⓇ Award and the iF Design Excellence Award.
**Early feasibility clinical study of investigational BD Libertas™ Wearable Injector (WI) evaluated 5 mL, non-Newtonian ~8cP subcutaneous placebo injections in 52 healthy adult subjects of ≥18.5kg/m2 BMI divided into 2 age groups (18-64 or ≥65 years) for functionality, tissue effects, subject tolerability and acceptability.
Access the full published journal article “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects” below.
Request article access
Let's have a conversation
Let's have a conversation
1. Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05
2. Design Output Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton Dickinson and Company; 2017 Revision: 04
3. ADDS Libertas Program Preclinical Study List BDTI Parenteral Sciences COE.
4. ADDS Libertas Program Clinical Study List BDTI Parenteral Sciences COE.
5. Woodley, W. D. et al. Clinical Evaluation of an Investigational 5ml Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021 May; 14(3):859-869. doi: 10.1111/cts.12946.
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.

