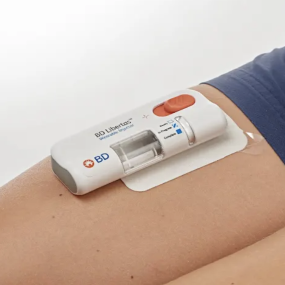
BD Libertas™ Wearable Injector
BD has conducted over 50 pre-clinical and clinical studies to inform the design and development of the BD Libertas™ Wearable Injector and demonstrating the feasibility of 2-10mL biologic injections into subcutaneous tissue. BD reinforced its commitment to innovation by completing a 52-subject human trial for its award-winning 5mL BD Libertas™ Wearable Injector where 100% of subjects reported that they were likely to use the injector if prescribed.*†,1
The 5mL and 10mL embodiments BD Libertas™ Wearable Injector share a common design architecture that incorporates configurable components to deliver a target dose volume and a range of injection times.
|
|
|
|||
| 5mL embodiment BD Libertas™ Wearable Injector |
10mL embodiment BD Libertas™ Wearable Injector |
BD Libertas™ Wearable Injector offers patients the simplicity of its Peel, Stick & Click™ design that does not require any patient filling or assembly. This design eliminates concerns around end-user filling or assembly related breakage, contamination and dosing errors.‡
The platform's fully mechanical operation does not require separate software or electronics supplier management and revision controls. Additionally, the BD Libertas™ Wearable Injector product format features a spring-based power source without battery or heavy metals disposal concerns.
Combined, these design decisions help reduce patient and pharma complexity and expand the possibilities for delivering biologic therapies outside the clinical environment.