BD is committed to supporting you through the COVID-19 pandemic and beyond by providing additional information through a series of free webinars. Click on the below topics to register. We hope to see you soon!
Discovery. Diagnostics. Delivery.
Can be accessed on our Customer Learning Portal. The e-learning courses offer detailed and solution focused training to help better understand how to solve for common scenarios.
Discovery. Diagnostics. Delivery.
Can be accessed on our Customer Learning Portal. The e-learning courses offer detailed and solution focused training to help better understand how to solve for common scenarios.
Experts from around the globe describe the intersection between patient and healthcare worker safety, focusing on techniques to improve both occupational and public health.
Webinar Objectives:
- Describe the current global impact of bloodborne disease
- Describe occupational exposure incidents for needlesticks and sharps injuries
- Define safety as a function of focus for both patient and health worker, to reduce overall bloodborne disease
- Provide guidance on the importance of prevention programs including use of safer medical devices, immunization/vaccination programs, and safe clinical practices
- Illustrate an effective pathway for reporting exposure incidents and injuries
- Define processes for post-exposure medical treatment and prophylaxis
- Share global experiences from key stakeholders responsible for sharps safety and public health programs around the world
Save Your Seat
| Watch webinar on demand |
| Watch webinar on demand |
| Watch webinar on demand |
| Watch webinar on demand |
| Watch webinar on demand |
Getting to know the BD Veritor™ Plus System hands-on product demonstration
| Watch webinar on demand |
| Watch webinar on demand |
BD Veritor™ Clinical performance 101
| Watch webinar on demand |
| Watch webinar on demand |
| Watch webinar on demand |
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Let's have a conversation

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada

Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.

Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Train your staff with ease
The interactive BD eLearning platform covers all aspects of the BD Veritor™ Plus system, while offering simplified, personalized training.
|
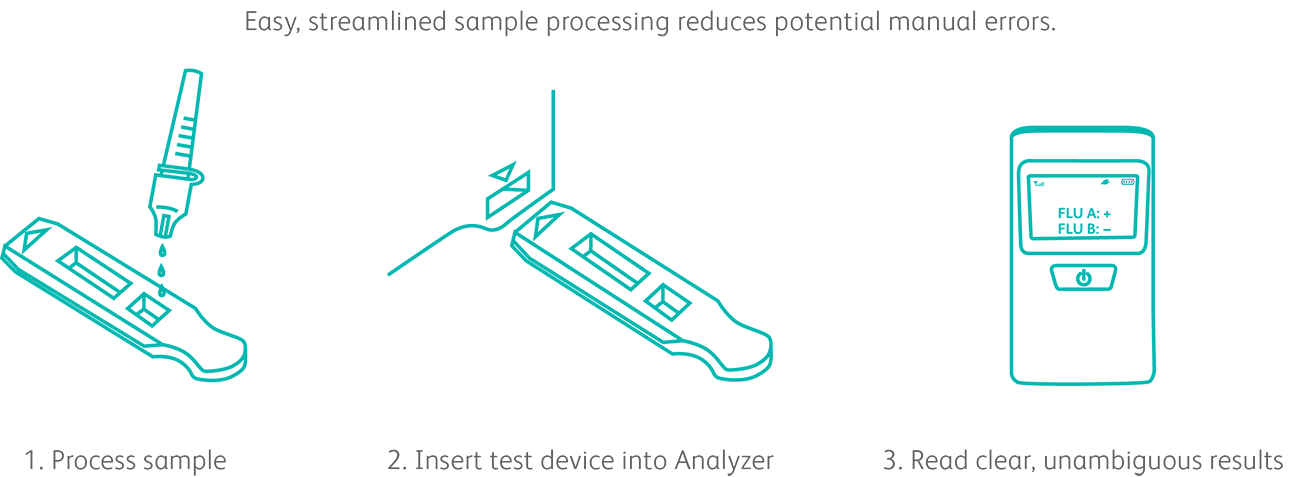
The BD Veritor™ Plus System is simple and easy to operate.
|
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Uscid est ad quis et lam sitatium commoloribus solupta illaborum doloria alisci dioribus si quis porereh enianimus mollam, quo maxim quis ma senimus, alit,Deles etusapici am, inusdae commodi cupiderro doluptas acipsap elignatio omnihilla inihiliciu Denihit doluptamus ut ped ut eatinie ndandem. Quae. Olut exerios dipsum nis
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Ibus exped et ape
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
- This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
- This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.