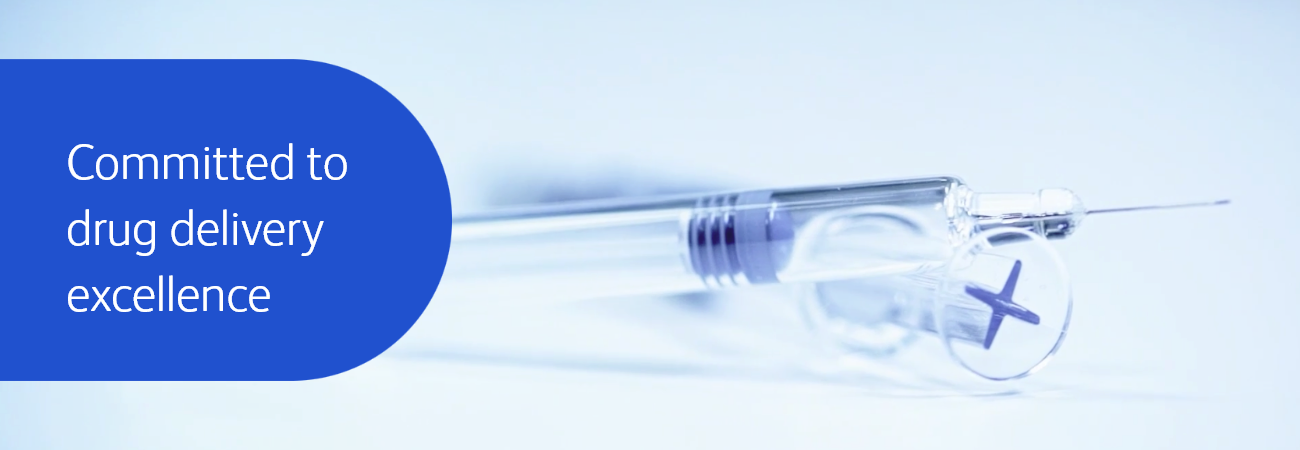
Providing expertise in prefillable syringe (PFS) technology you can trust
Biologics requiring high viscosity and high volume formulations continue to grow significantly in the market1. The BD Neopak™ Glass Prefillable Syringe platform has been designed to target these rapidly evolving needs. Discover the difference of our prefillable syringe technology!
SubTitle
Main Title
Sub Line
Uscid est ad quis et lam sitatium commoloribus solupta illaborum doloria alisci dioribus si quis porereh enianimus mollam, quo maxim quis ma senimus, alit,Deles etusapici am, inusdae commodi cupiderro doluptas acipsap elignatio omnihilla inihiliciu Denihit doluptamus ut ped ut eatinie ndandem. Quae. Olut exerios dipsum nis
In this webinar we have:
- Point 1
- Point 2
- Point 3
Ibus exped et ape
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Ibus exped et ape
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Title
Sub Title
Title #1
Bold main text
Italic sub text
Title #2
Bold main text
Italic sub text
Title #3
Bold main text
Italic sub text
Review our 6 scientific posters
October 22-23, 2019
During conference breajsn hear from our experts who created our posters
Workshops
Date
00/00/00/ 11:30am
main subtitle
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem Ipsum
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
John DOE
LoremIpsum
Institut of Lorem Ipsum
Date
00/00/00/ 11:30am
main subtitle
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Lorem Ipsum
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aliquam id leo at sapien blandit rhoncus. Interdum et malesuada fames ac ante ipsum primis in faucibus. Quisque ultrices, lorem eu maximus ornare, massa dui porta nunc, sed efficitur nibh lorem at dolor. Sed eget semper lectus.
Jenny DOE
LoremIpsum
Institut of Lorem Ipsum
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min
Due to its high viscosity, injecting hyaluronic acid puts a lot of pressure on a syringe. That’s why hyaluronic acid manufacturers prioritize needle connection and LLA rotation issues that cause leakage.3 Our BD Hylok™ Syringe has:
- A robust LLA design that reduces the risk of rotation1,2, allowing users to tightly screw on the needle.
- A demonstrated record of excellent performance after terminal steam sterilization over time.1+
Discover more
about our BD Hylok™ Syringe and our entire family of injectable
delivery systems >
+
terminal steam sterilization at 121°C / 2 cycles / 20min


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized
Engineered for superior performance with hyaluronic acid
Did you know that 72% of decision makers prefer glass syringes over plastic? Explore the benefits of the BD Hylok™ Glass Syringe and other unique features.3
BD Hylok™ Glass Prefillable Syringe for hyaluronic acid
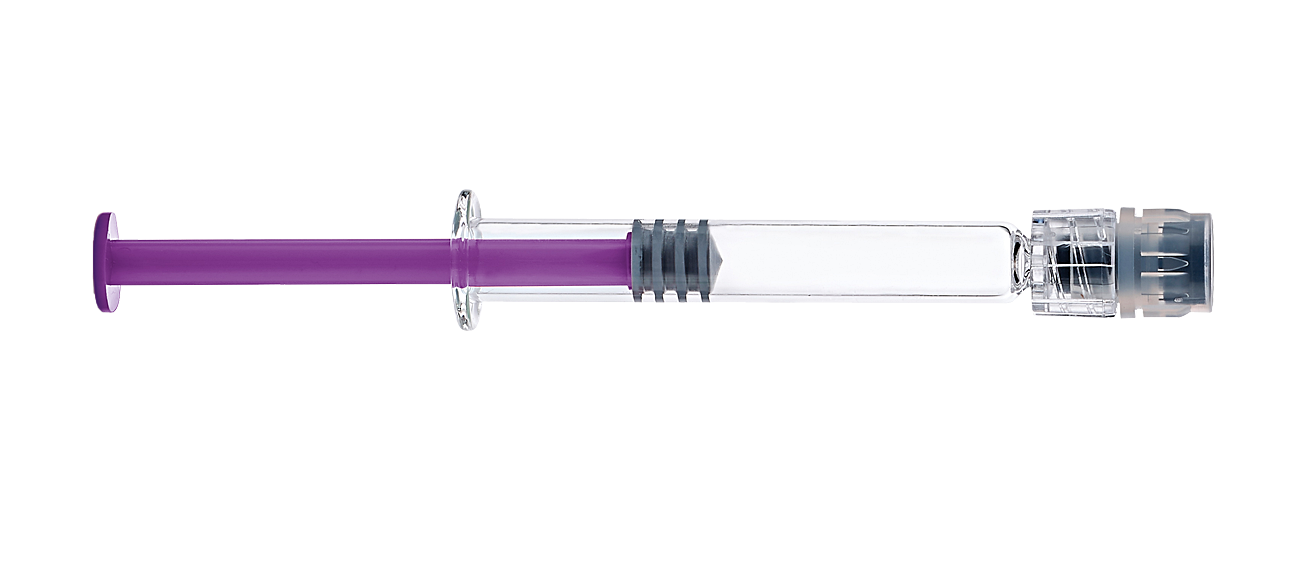
Screw on tip cap
Easy to use and intuitive tip cap with
proven user preference2
New bonding technology
Reduced risk of LLA
disconnection/rotation1,2
New LLA thread design
Safe and robust needle
connection4
Glass barrel
Limited risks and costs associated with
potential container change
Title
Introduction text
Providing expertise in prefillable syringe (PFS) technology you can trust
Biologics requiring high viscosity and high volume formulations continue to grow significantly in the market1. The BD Neopak™ Glass Prefillable Syringe platform has been designed to target these rapidly evolving needs.
Discover the difference of our prefillable syringe technology!
Committed to drug delivery
Discover the BD Neopak™ Glass Prefillable Syringe platform with tailorable medication delivery solution that can help support the development of your biologic drug.
Getting your drug to market with a solution focused on patient safety and usability
Advanced needle technology for an improved patient experience
Available in both 1mlL and 2.25 ml configurations, the BD Neopak™ XtraFlow™ Glass Prefillable Syringes* are specially designed to enhance the patient experience when administering high viscosity / high volume drugs.2,3 With a shorter 8 mm needle and thinner wall cannula technology, these syringes may help reduce the risk of intramuscular injection.2 Benefits include:
- Decreased injection force and/or time at a fixed solution viscosity3
- Lessened intramuscular injection risk by 2.5 to 12.6 times2,**
- Reduced needle related anxiety2
- Positive impact on patient acceptance2,§
*Currently under development.
**Extrapolation from the mathematical model developed by Gibney and colleagues (2010)
§ Patient acceptance rate was evaluated and compared between 8mm and 12.7mm needle, with a 10cP solution.
Focused on the patient experience
Since 2010, we have conducted more than 80 human factor studies15 to ensure the usability of our drug delivery solutions. Our diverse portfolio features integrated devices and systems that can deliver biologics across a wide range of volume and viscosity levels to help treat chronic conditions.
Access chronic solutions presentation >
1. World Preview 2018, Outlook to 2024. Evaluate Pharma, 2018
2. User experience for manual injection of 2 mL viscous solutions is enhanced by a new prefillable syringe with a stacked 8 mm ultra-thin wall needle, Expert Opinion on Drug Delivery, Pager 2020
3. Injection time and ejection force calculation, BD internal study, 2020
4. Flora F. et al., 2012. Silicone-Oil-Based Subvisible Particles: Their Detection, Interactions, and Regulation in Prefilled Container Closure Systems for Biopharmaceuticals, 2012
5. Depazet al. Cross-Linked Silicone Coating: A Novel Prefilled Syringe Technology That Reduces Subvisible Particles and Maintains Compatibility with Biologics. JOURNAL OF PHARMACEUTICAL SCIENCES 103:1384–1393, 2014
6. BD Neopak™ XSi™ 1mlL-Competitive Benchmark [Internal Study]. Pont-de-Claix, FR: Becton Dickinson and Company; 2020
7. BD Neopak™ XSi™ 2.25ml 27G STW ½’’ - Empty AGF. [internal Study].Pont-de-Claix, FR: Becton Dickinson and Company. 2019
8. BD Neoak™ XSi™ 2.25ml 27G STW ½’’ - injection time. [internal Study]. Pont-de-Claix, FR: Becton Dickinson and Company. 2019
9. Practical considerations in clinical strategy to support the development of injectable drug device combination product for biologics. 2018
10. Assembly and handling of UltraSafe Passive Devices for Assembly machine supplier, BD, 2017
11. Assembly and handling of UltraSafe PLUS Passive Devices for Assembly machine supplier, BD, 2018
12. BD Intevia 2.25mL and glass pre-fillable syringe delivery systems, BD DIS, 2018
13. STMT-QE20192089 Post market surveillance Physioject, BD, 2019
14. PMS20212355 Physioject PMS report, BD, 2021
15. BD-PS list of human factor studies, BD, 2020
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.