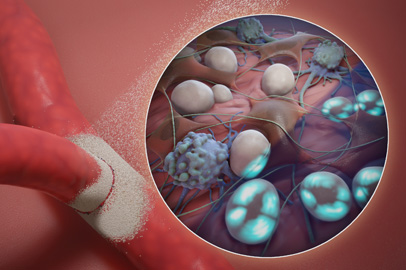
Manufactured from a purified plant starch,
Does not interfere with natural healing2,9
Arista™ AH used in a robotic-assisted total laparoscopic hysterectomy
Discovery. Diagnostics. Delivery.
Can be accessed on our Customer Learning Portal. The e-learning courses offer detailed and solution focused training to help better understand how to solve for common scenarios.
Experts from around the globe describe the intersection between patient and healthcare worker safety, focusing on techniques to improve both occupational and public health.
Webinar Objectives:
- Describe the current global impact of bloodborne disease
- Describe occupational exposure incidents for needlesticks and sharps injuries
- Define safety as a function of focus for both patient and health worker, to reduce overall bloodborne disease
- Provide guidance on the importance of prevention programs including use of safer medical devices, immunization/vaccination programs, and safe clinical practices
- Illustrate an effective pathway for reporting exposure incidents and injuries
- Define processes for post-exposure medical treatment and prophylaxis
- Share global experiences from key stakeholders responsible for sharps safety and public health programs around the world
Save Your Seat
Arista™ AH can provide a simple, safe and effective solution for intraoperative bleeding in urologic surgery procedures.1
![]()
Videos
Explore our library of technique videos demonstrating the effectiveness of Arista™ AH.
Watch Now
![]()
Case Study
Total laparoscopic hysterectomy surgery using Arista™ AH Absorbable Hemostatic Particles to control intraoperative bleeding.
View case study
![]()
Education
Access the BD Biosurgery educational programs, designed to provide you with in-depth education on the techniques and utilization of our hemostat and sealant portfolio of products.
View upcoming events
Arista™ AH
Arista™ AH is indicated in surgical procedures (except neurological and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.8
SURGICEL® Powder
Warning: SURGICEL® Powder is dry and there may be difficulties in precise delivery under certain circumstances. Unintentional device placement may result in powder scattering and device migration that may increase the risk of adhesion formation.9
HEMOBLAST™ Bellows
There is a risk of human parvovirus B19 transmission. Human parvovirus B19 most seriously affects pregnant women or immune-compromised individuals. It is not known whether HEMOBLAST™ Bellows can cause fetal harm when administered to pregnant women or can affect reproduction capacity.10
Let's have a conversation

Rapid tissue incorporation 1
Organized and functional collagen at the repair site1
4x the strength of the native abdominal wall at 24 weeks in preclinical testing1
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
Predictable strength for the long run
- Long-term strength with no permanent material left behind2,3
- Organized and functional collagen at the repair site3
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
The proprietary Arista™ AH powder achieves broad area coverage10 on rough surfaces and in hard-to-reach areas,9 for rapid control of bleeding in minutes.1
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Considerations when selecting a powdered hemostat1,19
| Arista™ AH Absorbable Hemostatic Particles¹ | PerClot® Polysaccharide Hemostatic System | ||
| Available sizes | 3 sizes: 1 g, 3 g, 5 g¹ | 3 sizes: 1 g, 3 g, 5 g² | |
| Economic | Shelf life | 5 year shelf life. | 3 year shelf life |
| Tip delivery system | FlexiTip™ XL applicator, includes (1) applicator (length: 38 cm) FlexiTip™ XL-R applicator, rigid, 38 cm FlexiTip™ applicator, includes (2) applicators (length: 14 cm).³ The FlexiTip™ / FlexiTip™ XL Applicator can be easily trimmed in the event that tip clotting occurs. It is important to use sharp scissors to avoid crushing the tip.³ |
PerClot® applicators are available in the following lengths: 100 mm and 200 mm (available for 3 g only.)² Do not attempt to trim the applicator tip. In the event that the tip becomes occluded, use a new applicator.² |
|
| Patient | Swelling | Warning: Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.¹ | Warning: Remove excess AMP® particles once hemostasis is achieved. This removal of excess particles is particularly important in and around the spinal cord, areas of bone confine, the optic nerve/chiasm, and foramina of bone because unsaturated particles may swell and compress the surrounding tissues.² |
| Absorption profile |
Arista™ AH absorption time: approximately 24–48 hours.¹* |
Absorption normally requires several days and is dependent on the amount of material applied and the site of use.² |
|
| Product | Device description |
Arista™ AH is a medical device intended for application to surgical wound sites as an absorbable hemostat. This technology incorporates hydrophilic, flowable, microporous particles synthesized by cross-linking purified plant starch through a proprietary process; Arista™ AH is a 100% plant-based polysaccharide.¹ |
PerClot® Polysaccharide Hemostatic System (PerClot® PHS) is a medical device composed of absorbable modified polymer (AMP®) particles and delivery applicators.² |
| Clinical study adverse reactions |
Per a randomized clinical study – there were no adverse events during clinical studies that were judged related to the device by the Data Safety Monitoring Board.¹‚4 | A total of seven adverse events have been reported for PerClot® PHS. Five adverse events were reported during clinical use. Three were potential re-bleeding resulting from the unidentified bleeding source during myomectomy, emergency epistaxis and septoplasty. One adverse event was reported for aspiration of dry particles into the airway during tonsillectomy. One adverse event was regarding broken applicator.² |
|
| Treatment of deep wounds |
Deep wounds may require equally deep application of Arista™ AH. To minimize occlusion of the tip, pressure should be applied to deliver Arista™ AH as the applicator enters the wound.¹ |
Precaution: Visualization of the bleeding site is critical during the application of PerClot® PHS. The bleeding site must be exposed to ensure the hemostatic particles contact with the bleeding site prior to achieving hemostasis, or else rebleeding may occur. Especially for its application in myomectomy, it is hard for PerClot® PHS to reach the actual bleeding site, so the hemostasis is not achieved.2 |
|
| Laparoscopic and endoscopic use |
Available with a FlexiTip™ XL-R applicator for endoscopic/laparoscopic procedures.¹ |
For specific surgical procedures, the system is configured in both PerClot® Standard and PerClot® Laparoscopic. (Available for 3 g only)² |
|
| Procedure | Cell saver compatible |
Arista™ AH is compatible with a 20–40µ size range for autologous blood salvage systems.¹ |
Precaution: When an extracorporeal cardiopulmonary bypass circuit or autologous blood salvage circuit is used in conjunction with PerClot® PHS, care must be exercised to prevent possible particle entry into the bypass circuit. Entry is prevented by using a 40µ cardiotomy reservoir, cell washing, and a 40µ transfusion filter (such as a LipiGuard®).² |
| ENT use | No warnings, contraindications or adverse events directly related to ENT use.¹ |
Warning: When PerClot® PHS is used in the nasal cavity and laryngopharyngeal, PerClot® PHS should be used with caution to avoid the dry particles being drawn into the trachea or bronchi, which may form a gel that blocks the trachea and bronchi.² |
|
| Urological procedures |
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.¹ |
PerClot® PHS should not be left in bladder, ureteral lumen or renal pelvis to eliminate the potential foci for calculus formation.² |
1. Based on Arista™ AH Instructions for Use: PK3798864.2015. 2. BD Data on file. Preclinical data may not correlate to clinical performance in humans. 3. Ereth MH, Dong Y, Schrader LM, Henderson NA, Agarwal S, Oliver WC, Nuttall GA. Microporous Polysaccharide Hemospheres do not enhance abdominal infection in a rat model compared with gelatin matrix. Surg Infect. 2009 Jun;10(3):273-6. 4. Hermans M, Brown L, Darmoc M. Adhesion prevention in an intraperitoneal wound model: Performance of two resorbable hemostats in a controlled study in rabbits. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100B(6):1621-1626. doi:10.1002/jbm.b.32730 5. Erdogan H, Kelten B, Tuncdemir M, Erturkuner S, Uzun H, Karaoglan A. Hemostasis vs. epidural fibrosis?: A comparative study on an experimental rat model of laminectomy. Neurol Neurochir Pol. 2016;50(5):323-330. doi:10.1016/j.pjnns.2016.05.002 6. Hoffmann N, Siddiqui S, Agarwal S et al. Choice of Hemostatic Agent Influences Adhesion Formation in a Rat Cecal Adhesion Model. Journal of Surgical Research. 2009;155(1):77-81. doi:10.1016/j.jss.2008.08.008 7. Poehnert D, Neubert L, Klempnauer J, Borchert P, Jonigk D, Winny M. Comparison of adhesion prevention capabilities of the modified starch powder-based medical devices 4DryField® PH and Arista™ AH in the Optimized Peritoneal Adhesion Model. Int J Med Sci. 2019;16(10):1350-1355. doi:10.7150/ijms.33277 8. Singh P, Vasques D, Deleon F. Microporous Polysaccharide Hemospheres for Adhesion Prevention: A Randomized Controlled Trial. J Gynecol Surg. 2013;29(4):196-202. doi:10.1089/gyn.2013.0007 9. Arista™ AH PMA Clinical Study, P050038 Approval date September 26, 2006 10. Bruckner In vitro study comparing MPH human blood to human blood without MPH In vitro studies may not be predictive of clinical outcomes. 11. Neveleff, D.J. Optimizing hemostatic practices: matching the appropriate hemostat to the clinical situation. AORN J 96, S1-S17 (2012). 12. Doria, C. & Vaccino, S. Topical hemostasis: a valuable adjunct to control bleeding in the operating room, with a special focus on thrombin and fibrin sealants. Expert Opin Biol Ther 9, 243-247 (2009). 13. Marietta, M., Facchini, L., Pedrazzi, P., Busani, S. & Torelli, G. Pathophysiology of bleeding in surgery. Transplant Proc 38, 812-814 (2006). 14. Shander, A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery 142, S20-25 (2007). 15. Boucher, B.A. & Traub, O. Achieving hemostasis in the surgical field. Pharmacotherapy 29, 2S-7S (2009). 16. Zimmerman, L.H. Causes and consequences of critical bleeding and mechanisms of blood coagulation. Pharmacotherapy 27, 45S-56S (2007). 17. Alstrom, U., Levin, L.A., Stahle, E., Svedjeholm, R. & Friberg, O. Cost analysis of re-exploration for bleeding after coronary artery bypass graft surgery. Br J Anaesth 108, 216-222 (2012). 18. Ye, X., Lafuma, A., Torreton, E. & Arnaud, A. Incidence and costs of bleeding-related complications in French hospitals following surgery for various diagnoses. BMC Health Serv Res 13, 186 (2013). 19. Based on 389894R04 – SURGICEL® Powder Instructions for Use (Code 3013SP) (Non-CE marked) 2017.
Arista™ AH is indicated in surgical procedures (except neurological and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
CONTRAINDICATIONS
Do not inject or place Arista™ AH into blood vessels as potential for embolization and death may exist.
WARNINGS
Arista™ AH is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
Once hemostasis is achieved, excess Arista™ AH should be removed from the site of application by irrigation and aspiration particularly when used in and around foramina of bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm. Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. Dry, white Arista™ AH should be removed. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.
Safety and effectiveness of Arista™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
v Arista™ AH should be used with caution in the presence of infection or in contaminated areas of the body. If signs of infection or abscess develop where Arista™ AH has been applied, re-operation may be necessary in order to allow drainage.
Safety and effectiveness in neurosurgical and ophthalmic procedures has not been established.
Arista™ AH should not be used for controlling post-partum bleeding or menorrhagia.
PRECAUTIONS
When Arista™ AH is used in conjunction with autologous blood salvage circuits, carefully follow instructions in the Administration section of the IFU regarding proper filtration and cell washing.
Arista™ AH is intended to be used in a dry state. Contact with saline or antibiotic solutions prior to achieving hemostasis will result in loss of hemostatic potential.
Arista™ AH is not recommended for the primary treatment of coagulation disorders.
No testing has been performed on the use of Arista™ AH on bone surfaces to which prosthetic materials are to be attached with adhesives and is therefore not recommended.
Arista™ AH is supplied as a sterile product and cannot be resterilized. Unused, open containers of Arista™ AH should be discarded.
Do not apply more than 50g of Arista™ AH in diabetic patients as it has been calculated that amounts in excess of 50g could affect the glucose load.
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
ADVERSE REACTIONS
None of the adverse events that occurred in a randomized prospective, concurrently controlled clinical trial were judged by the Data Safety Monitoring Board to be related to the use of Arista™ AH. The most common recorded adverse events were pain related to surgery, anemia, nausea, lab values out of normal range, arrhythmia, constipation, respiratory dysfunction and hypotension – all reported in greater than 10% of the Arista™ AH treated patients. The details of this clinical trial’s adverse events can be reviewed in the IFU supplied with the product and are also available at www.bd.com.
Caution: Federal (USA) law restricts this device to sale by or on order of a licensed physician or properly licensed practitioner.
BD-71804
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.